The alternating access mechanism in Mhp1
Secondary transporters couple the free energy stored in an ionic gradient to the movement of solutes across the cell membrane. The coupling enables these transmembrane proteins to transport small molecules against their own concentration gradients. The transporters function by cycling between different conformational states in which access to the central binding site is switched from the extracellular solution to the intracellular compartment. This abstract alternating access model was proposed by Oleg Jardetzky in 1966 (Nature 211 (1966), 969–970) but its structural basis was not established for any transporter family.
According to the model, a cation-substrate symporter would go through a cycle of a number of distinct conformations:
- In the outward-facing open conformation, the substrate and ions can enter the binding sites from the extracellular space. The binding site is located near the centre of the protein.
- A conformational change takes place that closes access to the outside. Ion and solute are sealed off in this occluded state.
- Then protein switches to the inward-facing open conformation. The binding sites become connected to the intracellular compartment and ion and solute are released.
- The cycle is closed by a transition back to 1 in the absence of substrate. Whereas the transitions 1→2 and 2→3 are coupled to the ionic gradient's free energy, the transition 3→1 must be able to occur spontaneously through activation by thermal fluctuations.
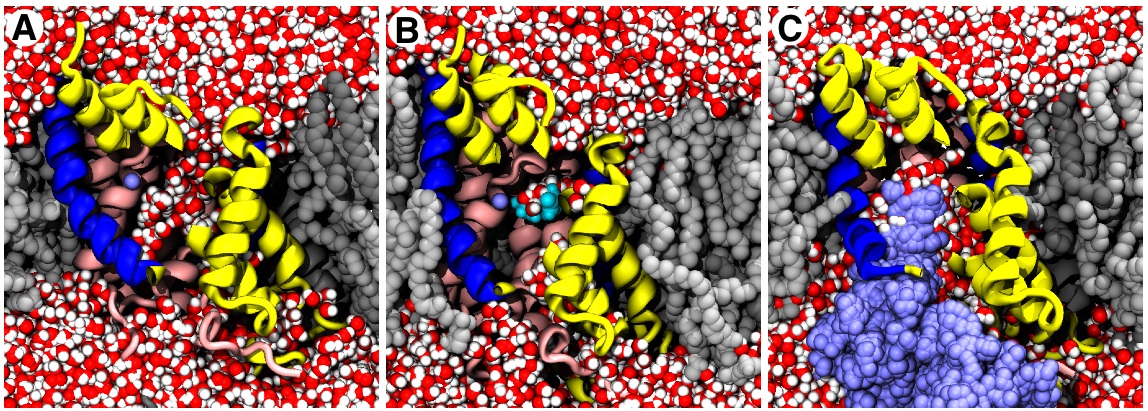
We studied the hydantoin permease from Microbacterium liquefaciens, a sodium-coupled nucleobase symporter and a member of the NCS1 family. Mhp1 shares the same general architecture with other transporters such as the sodium-amino acid transporter LeuTaa (which is a bacterial homolog of the important mammalian neurotransmitter:sodium symporter (NSS) family). Weyand et al. solved the two outward-facing conformations of Mhp1 in 2008. In 2010 we determined the inward facing conformation (Shimamura et al (2010)) and carried out computer simulations of Mhp1 in all three conformations. Using a novel simulation method (dynamic importance sampling) we were also able to simulate the conformational transition itself.
Based on the combination of structural data and simulations (movie in MPEG4 format) we could identify the moving elements of the transport mechanism and propose a sequence of events that leads to solute transport as shown in the animated cartoon.
Our hypothetical model for secondary transport in Mhp1 contains the
following steps and describes the transporter as a triple-gated
pore
:
- In equilibrium and in the absence of substrate and ions, the protein switches spontanously between the inward facing and the outward facing state.
- Once Na+ binds to the outward facing state, it stabilizes it and allows the substrate (here benzyl-hydantoin) to bind, too.
- The extracellular thin gate closes.
- The thick gate switches from outward facing to inward facing.
- The intracellular thin gate opens and substrates are released to the intracellular space.
- Go back to 1.
By utilizing a system of (synchronized) gates, the protein appears to prevent the formation of a continuous conduction pathway, which would lead to uncoupled leakage of Na+ into the cell or loss of substrate out of the cell. In other words, our model describes in molecular detail a transport process that has the properties required by the alternating access mechanism.
For more details see the paper Shimamura et al. (2010).