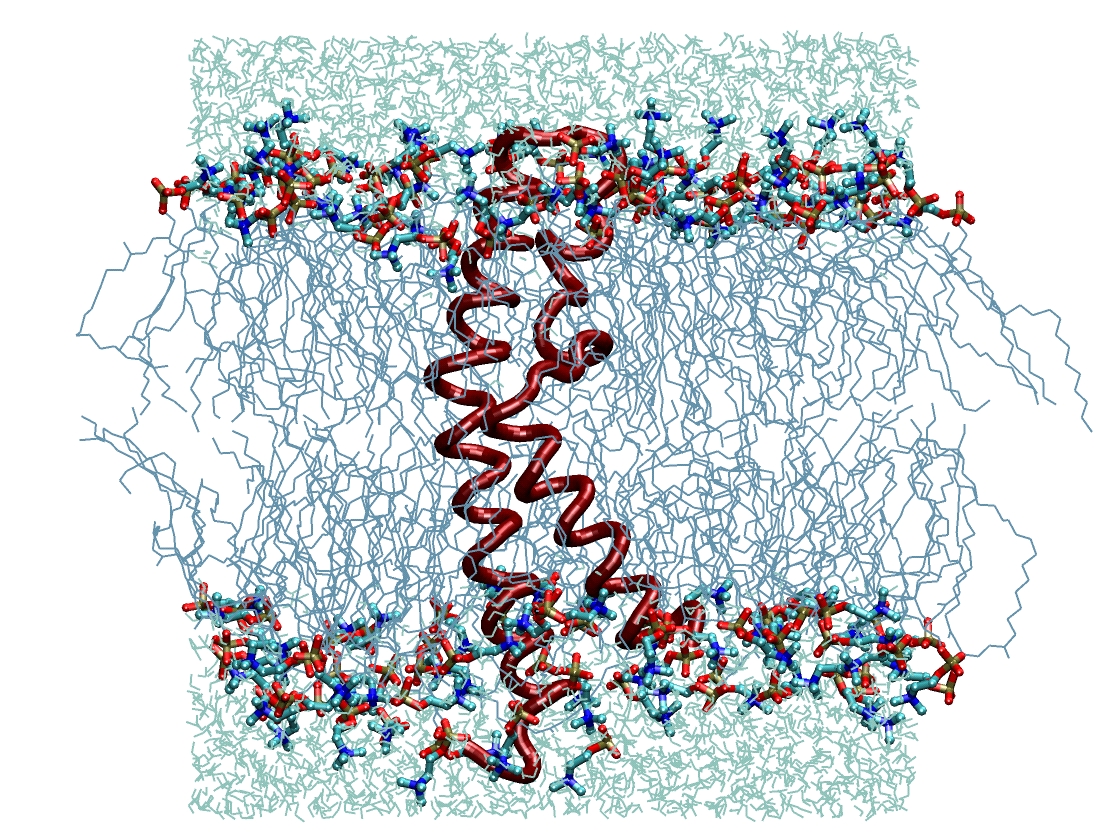
Molecular Dynamics Simulations of the Escherichia coli H+F0F1 ATP Synthase Transmembrane c Subunit.
The transmembrane c subunit is the H+-translocating component of the F0F1 ATP synthase complex. Protonation and deprotonation of the specific aspartic acid involved in proton transport leads to structural changes. Based on solution NMR structures, Molecular Dynamics simulations are used to compare the structural and dynamics properties of the monomeric Escherichia coli c subunit in a lipid bilayer of 1-palmitoyl-2-oleoyl-3-sn-phosphatidylcholine (POPC) at different protonation states of the aspartic acid. Two double mutants, A24D/D61N and A20P/P64A, have also been studied in order to investigate the role of the aspartic acid and of the proline, as well as the importance of their position in the helix. Simulations reveal the instability of a charged residue in a hydrophobic environment and support the proposed nechanism of protonation-deprotonation of the carboxyl group during translocation cycle. Analyses of kink angle and helical flexibility support an anisotropic behaviour around the proline, as seen in other proline-distorted transmembrane α-helices.

|