|
Ionotropic glutamate receptors (iGluRs) are responsible for most of the fast synaptic neurotransmission in the central nervous system (CNS) and are thought to play an important role in memory and learning in particular. The iGluRs also seem to be associated with many neurological diseases (including Alzheimer's, Parkinson's disease, stroke, epilepsy, amyotropic lateral sclerosis and schzophrenia) and as such represent important drug targets. They can be classified (See figure on the right) into subfamilies according to their affinities for the non-physiological agonists, NMDA (N-methyl-D-Aspartate), AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) and kainate. There is also a fouth sub-family; the delta subunits which are believed to incapable of forming functioning receptors on their own. | 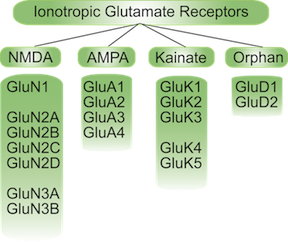 |
|
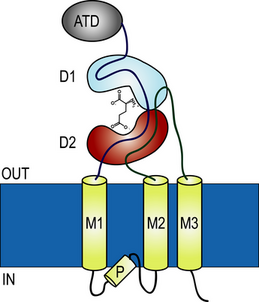
The basic topogy of each subunit can be described by the following diagram. There is a now complete structure of a GluA2 tetramer (see Sobolevsky et al (2009) and also a large
series of x-ray structures for the ligand-binding domains of members of each subtype family.
(see Mayer and Armstrong (2004) for a review).
We are using these structures (as exemplified in the jmol figure on the right of the schematic below) as a starting point to investigate the dynamics of these receptors.
|
 |
|
Our results (Arinaminpathy et al,
2002) indicated how the dynamics of the protein appear to be correlated with degree of cleft closure which has implications for the efficacy of the agonist
bound. We have extended that work to related receptors and proteins (see for example
Pang et al, 2003 and
Pang et al, 2005). We have also examined the role of ions in the interface in the kainate receptor
subfamily (Plested et al, 2008 and Vijayan et al,
2009)
and more recently the role of water in the binding pockets of this receptor family (Vijayan et al, 2010 and
Sahai & Biggin, 2011).
|
|
Nicotinic acetylcholine receptors (nAChRs)are another class of ligand-gated ion channel. In this case the natural ligand is acetylcholine. Nicotinic receptors are part of a gene superfamily of ligand-gated ion channels that includes receptors for glycine, 5-hydroxy-tryptamine (5-HT3 receptors) and gamma amino butyric acid. AChRs are distributed in two distinct areas in the body. The first is at the neuromuscular junction where they are responsible for converting the signal from spinal motor neurons to contraction of skeletal muscle. These AChRs are the best characterized in part due to the fact that it is these receptors that are found in large amounts at the modified neuromuscular juntion of electrical eels and have thus provided an easily accessible source for structural studies. The second type of AChR is found in the CNS but plays a relatively minor role compared to the glutamate receptors. Here the AChRs are thought to modulate neurotransmission rather than carry it per se. NAChRs are of course the target for the addictive component of tobacco, nicotine, which causes millions of people to die from smoking related diseases every year. These receptors are also implicated in other diseases including schizophrenia, rare forms of epilepsy and (through loss of AChRs) Alzheimer's and Parkinson's diseases. Each receptor is made up of five polypeptide chains. Each chain can either be the same (thus making a homomeric receptor) or it can be different (heteromeric). Depending on how similar each chain is to another chain they can be further classified into subtypes. Subtypes are labelled by greek characters,so we have α, β, γ etc. Although one might think that this might lead to massive number of different receptors, in fact certain combinations appear to predominate (for example a common nAChR found in the brain is the (α4)2(β2)3. Whatever the combination is, there must be two α subunits as they are essential for making a correctly formed binding pocket.
|
| One of the best studied nAChRs is the homopentameric (α7)5, which is a neuronal receptor. Although we do not have a full-length structure for this receptor, we do have a structure of the heteromeric nAChR from Torpedo thanks to the EM work of Nigel Unwin. This in combination with a series of crystal structures of the related acetylcholine binding proteins from snails (Celie et al. 2005b; Celie et al. 2005a; Celie et al. 2004 Brejc et al. 2001; ) can be used to model human receptors. Our research can be divided into the following sections: 1. Construction of full-length receptor models and subsequent analysis of dynamic properties. We have developed a general procedure that can be used dock two proteins together in an unbiased fashion which we have used to build an α7 receptor based on the achbp structures for the ligand binding domain and the EM data for the transmembrane region. This has recently been published, and is the model shown on the right 2. Use of these models to aid drug-design. |
